Why ideal soil pH increases yield and how to achieve it
As in many problems growers encounter, deviations from ideal soil pH should be tackled early to prevent yield loss. Ideally, growers should adjust the soil pH before planting as adjustments when plants in the soil are slower and put the plants at risk.
What is pH
Soil pH is a measure of the concentration of hydrogen ions in the soil. It is measured on a scale from 0 to 14, with 7 being neutral. When the pH is below 7, the medium is acidic, while above 7 is called alkaline.
How does the soil pH impact plants and what is the ideal soil pH
The pH value has a considerable effect on which nutrients plants can extract from the soil. Chemical reactions in the soil might tie nutrients up when the pH is not in the desirable range. It is common, for example, to see symptoms of chlorosis in new foliage even when fertilizers are provided regularly. Many times the reason is a high pH that makes the micro-elements less available to plants. At low pH, the leaching of magnesium and potassium increases, and their availability in the top layer of the soil decrease.

The most accessible form of zinc, manganese, and copper to plants require soil with a pH level between 5.5-6.5, although they can still be utilized at a moderate rate in a wider range of 4.5-7.5.
Iron becomes moderately available when the pH is below 7.5 and is highly available below 6.5. Other nutrients become less available when the pH is very low. Note that FeEDDHA (ethylenediamine-di-o-hydroxy-pheny lacetic acid), a type of chelated iron that is also known as Sequestrene 138, is available to plants even if the pH reaches 9. This is not the case with iron sulfate; another compound used to treat iron deficiency. But using FeEDDHA is not a long-term solution for iron deficiency.
In general, chelated fertilizers work better compared to regular micronutrients when the soil pH is greater than 6.5.
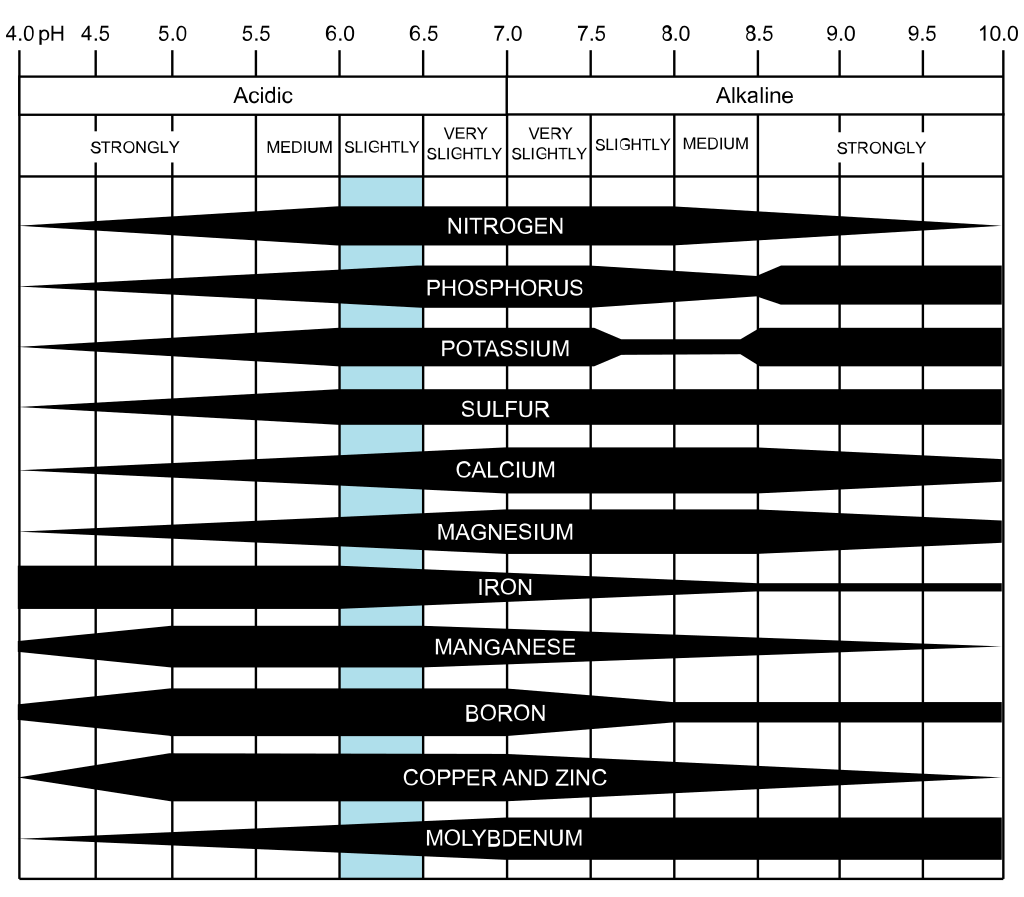
Heavy metals that are toxic to plants are more mobile in acidic soils and therefore are more likely to be taken up by plants. Aluminum is one of the metals that dissolve in low pH and damage plants. Severe acidity harms essential microorganisms and causes damage to the soil structure.
Since the optimal pH depends on the grown type of plant, we are providing a table with common crops and pH targets below.
| Plant | Optimal pH range |
|---|---|
| Alfalfa | 6.6-7.0 |
| Corn | 5.8-6.2 |
| Soybean | 6.3-6.5 |
| Wheat | 6.3-6.5 |
| Rice | 5-6.5 |
| Guava | 5.5-6.8 |
| Most vegetables | 6.0-6.8 |
| Citrus | 6.0-6.8 |
| Coffee | 5.0-6.0 |
| Blueberry | 4.5-5.5 |
| Rose | 6.0-6.5 |
To conclude, growers should aim to obtain ideal soil pH in the range that is most suitable for their plants to optimize the availability of the entire spectrum of nutrients and increase yield.
How the soil pH can change with time
The pH level in the soil can change due to fertilizers or the addition of organic matter such as compost and by watering with either acidic or alkaline water. When measuring the soil pH, it is essential to check the pH of the water as well. A non-neutral water pH will make the soil pH unstable.
Organic matter and minerals that break down in the soil over time lower the soil’s pH. In addition, nitrate that goes below the root zone (nitrate leaching) causes the pH to decrease as well. Therefore growers should constantly plan ahead to keep the pH in a range that supports plant growth. Soil pH intervention takes time to have an impact, and therefore planning, and early actions are crucial.
With poor soil drainage, you can expect an accumulation of salts in the field and an increased pH. Increasing the soil calcium concentrations can help with improving drainage. As calcium concentration goes up, the soil porosity improves.
How to measure soil pH and when to do it
The soil pH can be tested in the lab after soil samples from different parts of the field or garden are sent. In addition, there are field kits that provide a colorimetric indicator that show an estimate of the pH. Different kits require mixing the soil with water before the measurement.
When measuring the pH in the field, water the soil first. Use distilled water to make the soil wet and avoid making it muddy. To make the measurement accurate, take a sample of the soil away from spots exposed to direct sunlight (especially on hot days).
A more convenient way to measure pH is to use a digital soil ph meter. In this case, examine the probes and make sure they are in good condition and rust-free. Calibrate it with natural water before performing the test. Wash the probes with distilled water to remove salts before measuring.

How to change the soil pH
When the pH measurements start approaching the edges of the ideal soil pH range, it is time to act. Ideally, you should apply corrections in the fall, well before the planting, as it takes time for the pH to change.
How to lower the soil pH
To lower the pH, sulfur should be applied to the soil. Elemental sulfur, aluminum sulfate, and sulfuric acid are some of the options. The different forms vary in costs and speed of action. Elemental sulfur is considered a safe and economical way to reduce soil pH and is highly recommended to home growers.
If there are already plants in the soil, apply sulfur in low doses once a month. High dosages of sulfur are toxic to plants. Test the pH again every month.
The dosage depends on the soil type and the gap between the starting point and target pH. Soils rich in clay or organic matter, for example, have a high buffering capacity and require more sulfur before change can be observed.
Sulfur should be incorporated into the soil to affect the layer in the soil that is penetrated by the roots. Watering the soil after the sulfur application can help with that.
Gypsum (calcium sulfate) is another option if you grow in alkali soil. It is not very soluble, and therefore in normal soils, it will have a very minimal effect. In alkali soil, however, the sodium concentration is high. The calcium in the gypsum replaces the sodium in chemical interactions. This results in increased water infiltration and lowering of the soil pH.
Note that organic matter such as peat moss that has a pH of 3.0–4.0 can lower the soil pH as well.
As long as the pH is above 6.5, provide the micro-elements in foliage spraying.
How to increase the soil pH
Lower soil acidity can be obtained by adding lime to the soil. Ground agricultural limestone is a popular choice. The pace of change will be higher as finer the limestone particles. Hydrated pulverized limestone is considered to have the fastest effect, but precise dosages are important and can be easily missed.
Avoid using calcium magnesium carbonate (dolomitic limestone) if the soil contains normal or high magnesium levels. Adding magnesium to the soil in such situations can be toxic to the plants.
To calculate the amount of lime needed, refer to the soil test. The buffer capacity (ECEC) is the soil’s ability to resist pH changes. ECEC measures the Cation exchange capacity (CEC), a measure of the soil’s ability to hold positively charged ions. The buffer capacity, together with the desired change in pH, dictates the amount of lime that should be added.
The lime should be incorporated into the soil to a depth of at least 10 cm. Otherwise, the effect will be very superficial, and the deep soil layers will stay acidic. Optimal soil pH correction is the one that results in uniform acidity along the soil profile. Due to that, growers that avoid the destruction of the soil structure might find it challenging to optimize the pH profile.
Summary
The soil pH is a crucial factor when it comes to plant survival and prosperity. Farmers and home growers should monitor the soil pH constantly and plan to keep it in the optimal range. We are looking forward to welcoming you to our community and supporting you with any questions you might have regarding soil pH and plant health. In the meanwhile, as always, we wish you an abundant harvest.
